Abstract
Background. Multiple myeloma (MM) is an incurable malignancy of mature plasma cells. Despite major advances in the therapeutic landscape of MM, only 50.7% of the patients survive more than 5 years after diagnosis, with significantly lower rates (21%) for high-risk patients. Immunotherapy targeting BCMA is a promising MM treatment. However, BMCA is heterogeneously expressed in myeloma cells, leading to clonal selection of BCMAlow/negative cells under anti-BCMA therapies. Further, BCMA is often down-regulated leading to disease relapse, a mechanism called antigen escape and, it is expressed in normal cells causing on-target/off-tumor toxicity. Lastly, most patients have only transient disease remissions after anti-BCMA immunotherapy that do not last more than 18 months. Thus, we developed a target discovery pipeline in MM to identify novel and suitable immunotherapeutic targets.
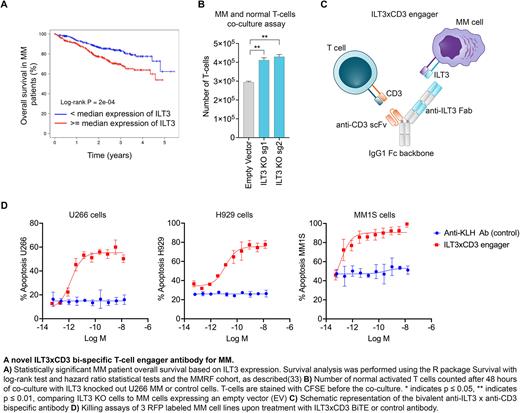
Methods. We run an unbiased mapping of the MM surface proteome with Mass-Spectrometry (MS) and 900+ patient samples. We found that ILT3 (aka LILRB4) is highly expressed in MM cells including primary samples from patients who relapsed post BCMA Chimeric Antigen Receptor (CAR) T-cell therapy, that we used for validation. We further validated ILT3 expression in primary normal hematopoietic stem and T cells by flow-cytometry and many normal tissues of the whole body. We performed survival analysis in 767 newly-diagnosed MM patients, including high-risk patients bearing the t(14;16) translocation compared to patients with no genetic abnormalities. Functionally, we knocked-out ILT3 in MM cell lines with the CRISPR/Cas9 technology and performed co-culture assays with normal T cells for 48 hrs at an Effector:Target (E:T) cell ratio of 2:1, to investigate potential impact on T cell proliferation. Given the promising results in terms of expression profiles of ILT3 and its biological relevance in MM, we developed a novel bi-specific T cell engager targeting ILT3 (ILT3 x CD3). The engager is a bivalent scFv-Fab bispecific on an effectorless human IgG1 backbone with a knob-in-hole design. We performed in vitro killing assays in which human T cells were combined with red fluorescent protein (RFP)-labeled MM cells at an E:T ratio of 5:1 in the presence of a dose titration of the bispecific, and induction of apoptosis was measured by activation of caspase 3/7 in target cells using a live imaging system. Further, we treated NSG immunocompromised mice injected with human MM cells including BCMA-KO MM cells and normal T-cells at a ratio of 2:1 and measured serum concentrations of human kappa light chains levels as a readout of tumor burden under treatment with the ILT3xCD3 antibody compared to mice treated with control IgG. In vivo treatment was performed intravenously 7 days after cell injection and once a week for four weeks and tumor tissue infiltration and overall mice survival were assessed.
Results. ILT3 is an immunoreceptor tyrosine-based inhibition motif-containing receptor and a marker of monocytic acute myeloid leukemia (AML). An antagonist antibody has been recently FDA-approved for treatment of AML. We found that ILT3 is highly expressed in MM cell lines bearing high-risk cytogenetics by MS analysis and validated ILT3 protein expression in 30+ primary patient samples with relapsed/refractory MM. Further, we validated absent ILT3 expression in primary HSCs and T cells. We also found that ILT3 significantly impacts clinical outcomes of MM patients. Functionally, we found that KO of ILT3 promoted an increase in T-cell numbers associated with a reduction of MM cells in co-culture. Thus, we generated an ILT3-targeting T cell engager that showed potent effects in triggering T cell-dependent cytotoxicity against multiple MM cell lines including BCMA-KO cells in vitro and in vivo, with EC50 values in the low pM range. Treatment with ILT3xCD3 engager decreased MM tumor burden and prolonged mice survival.
Conclusions. Our work represents the first evidence and rationale for clinical development of NGM936 surrogate antibody an ILT3-targeted immunotherapy as a viable therapeutic approach for MM cell killing. These data suggest that targeting ILT3 is an effective and safe therapeutic approach in MM.
Disclosures
Guzman:BridgeMedicines: Research Funding; Samus Therapeutics: Other: Inventor on licensed IP; Seq RX: Current holder of stock options in a privately-held company. Perna:Lonza: Research Funding; NGMBio: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal